Ad Search Design Control Medical Devices. Garwood Medical Devicess latest funding round in August 2020 was reported to be 4 m.
 Garwood Medical Devices Raises 3m In Series B Funding Finsmes
Garwood Medical Devices Raises 3m In Series B Funding Finsmes
Buffalo NY-based Garwood Medical develops the BioPrax device for minimally invasive procedures for eliminating biofilm infections on prosthetic joints during early intervention procedures.

Garwood medical devices. Garwood Medical Devices GMD LLC is a medical device company with a core mission. At Garwood were not just innovators. They are clinician programmable providing treatment while remotely monitoring operating parameters patient biometrics and compliance.
Expect in medical device manufacturing development shipping and quality management. Were fascinated by how things workand by making these advances even better. Ad Search Design Control Medical Devices.
They will begin trials of their BioPrax device a minimally invasive device used to treat infections on knee implants. Expect in medical device manufacturing development shipping and quality management. To Advance Infection Control Through Innovation.
These technologies are currently under investigation for FDA. Garwood Medical Devices LLC. Get Results from 6 Engines at Once.
Granted FDA Breakthrough Device Designation for products that may provide more effective treatment or diagnosis of life-threatening or irreversibly debilitating diseases or conditions In vivo animal and in vitro lab studies demonstrated up to 996 effectiveness in killing biofilms including drug-resistant bacteria such as MRSA E. Garwood Medical Devices has been granted Breakthrough Devices designation from the US. Food and Drug Administration FDA for the companys BioPrax device.
Ad Cev provides products and clinical solutions to all markets across the world. In total Garwood Medical Devices has raised 113 m Garwood Medical Devices Capital Raised. Food and Drug Administration FDA for the companys BioPrax device.
To Advance Infection Control Through Innovation Garwood has patented technologies that provide effective. Garwood Medical Devices has been granted Breakthrough Devices designation from the US. Garwood Medical Devices GMD is a medical device company with a core mission.
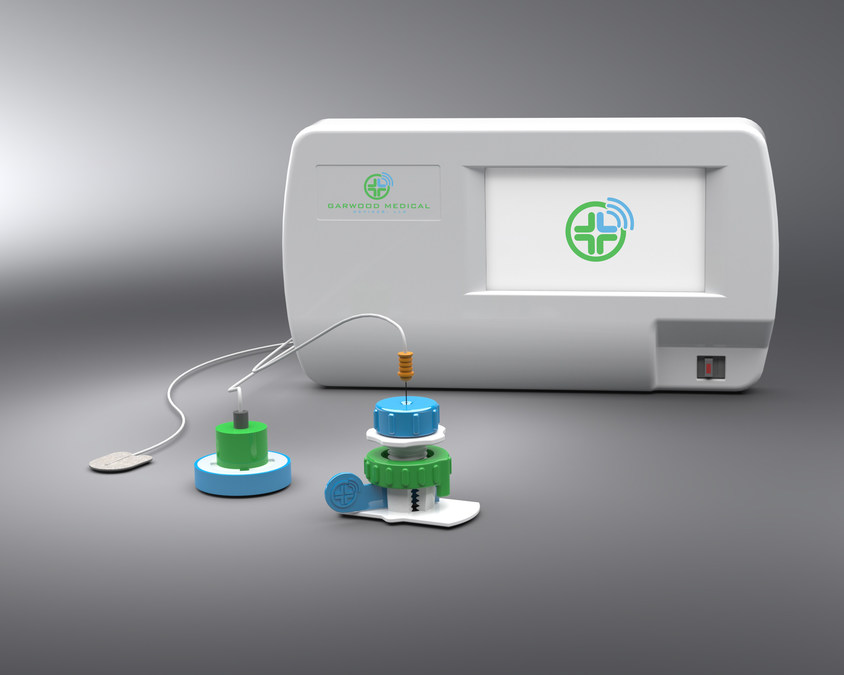
Garwood Medical Devices LLC GMD founded July 15 2014 is a medical device company developing a new class of active remotely powered medical devices. Ad Cev provides products and clinical solutions to all markets across the world. The Biofilm Disruption Device BDD is a minimally invasive electroceutical medical device that can be used on any metal implant.
Our intention is to provide a solution to help all patients suffering from bacterial biofilm infections by utilizing our cutting-edge technologies that will one day have the potential to enhance the current standard of care with the goal being to allow patients to retain the implants. Garwood has patented technologies that provide effective efficient and measurable electrical stimulation treatments for chronic wound healing bone growth and peri-prosthetic infection. Stay up to date on our latest developments as we strive to change the world one patient at a time.
BioPrax is a minimally invasive and cost-effective tool that is being developed to help eliminate biofilm infections on prosthetic knee implants during early intervention procedures while also maintaining the current. The companys devices utilizes a biofilm disruption technology to make infection-causing bacteria visible to the immune system and antibiotics by breaking down the biofilm on implants in order to disrupt before. Garwood Medical Devices is a developer of electronic medical devices intended to eliminate the need for follow-up surgeries.
It utilizes low voltage electrical current in a two-hour treatment that breaks up biofilms kills bacteria and stops infections early on before they become problematic. Garwood Medical Devices is a six-year old company which spun off UB research. Stay up to date on our latest developments as we strive to change the world one patient at a time.
BioPrax is a minimally invasive and cost-effective tool that is being developed to help eliminate biofilm infections on prosthetic knee implants during early intervention procedures while also maintaining the current standard of care. February 26 at 208 PM. Get Results from 6 Engines at Once.
Thats also been a motivating force throughout the career of our VP of Engineering and CTO Brian Peterson.
 Garwood Medical Devices Announces 4 Million Series C Funding Covering The Specialized Field Of Orthopedic Product Development And Manufacturing
Garwood Medical Devices Announces 4 Million Series C Funding Covering The Specialized Field Of Orthopedic Product Development And Manufacturing
 Garwood Medical Devices 3m Series B Oversubscribed By 700 000
Garwood Medical Devices 3m Series B Oversubscribed By 700 000

 Garwood Medical Devices Innovation Hub University At Buffalo
Garwood Medical Devices Innovation Hub University At Buffalo
 Garwood Medical Looks Ahead To Clinical Trials Through Ub Business Buffalonews Com
Garwood Medical Looks Ahead To Clinical Trials Through Ub Business Buffalonews Com
 Garwood Medical Looks Ahead To Clinical Trials Through Ub Business Buffalonews Com
Garwood Medical Looks Ahead To Clinical Trials Through Ub Business Buffalonews Com
 Garwood Medical Devices Announces Series C Funding
Garwood Medical Devices Announces Series C Funding
 Could Electrical Stimulation Reduce Joint Replacement Infections Medical Design And Outsourcing
Could Electrical Stimulation Reduce Joint Replacement Infections Medical Design And Outsourcing

 Garwood Medical Devices Crunchbase Company Profile Funding
Garwood Medical Devices Crunchbase Company Profile Funding
 Gregg Gellman Teaming Up With The Best In The Medical Device Business
Gregg Gellman Teaming Up With The Best In The Medical Device Business


 Garwood Medical Raises 4m In Series C Round Massdevice
Garwood Medical Raises 4m In Series C Round Massdevice
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.